publications
2024
-
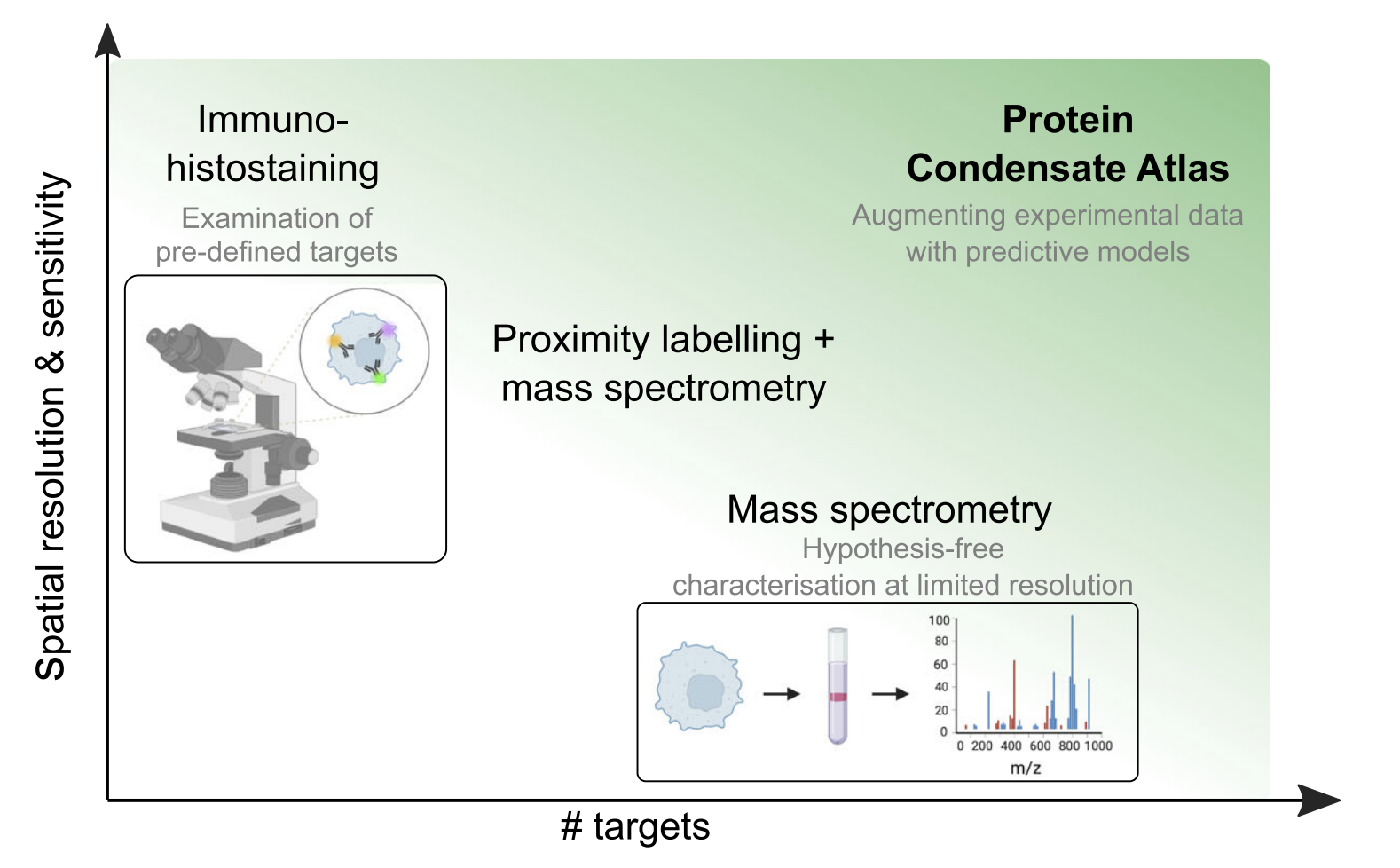 Protein condensate atlas from predictive models of heteromolecular condensate compositionK. L. Saar, R. M. Scrutton, K. Bloznelyte, A. S. Morgunov, and 4 more authorsNature Communications, 2024
Protein condensate atlas from predictive models of heteromolecular condensate compositionK. L. Saar, R. M. Scrutton, K. Bloznelyte, A. S. Morgunov, and 4 more authorsNature Communications, 2024Biomolecular condensates help cells organise their content in space and time. Cells harbour a variety of condensate types with diverse composition and many are likely yet to be discovered. Here, we develop a methodology to predict the composition of biomolecular condensates. We first analyse available proteomics data of cellular condensates and find that the biophysical features that determine protein localisation into condensates differ from known drivers of homotypic phase separation processes, with charge mediated protein-RNA and hydrophobicity mediated protein-protein interactions playing a key role in the former process. We then develop a machine learning model that links protein sequence to its propensity to localise into heteromolecular condensates. We apply the model across the proteome and find many of the top-ranked targets outside the original training data to localise into condensates as confirmed by orthogonal immunohistochemical staining imaging. Finally, we segment the condensation-prone proteome into condensate types based on an overlap with biomolecular interaction profiles to generate a Protein Condensate Atlas. Several condensate clusters within the Atlas closely match the composition of experimentally characterised condensates or regions within them, suggesting that the Atlas can be valuable for identifying additional components within known condensate systems and discovering previously uncharacterised condensates.
-
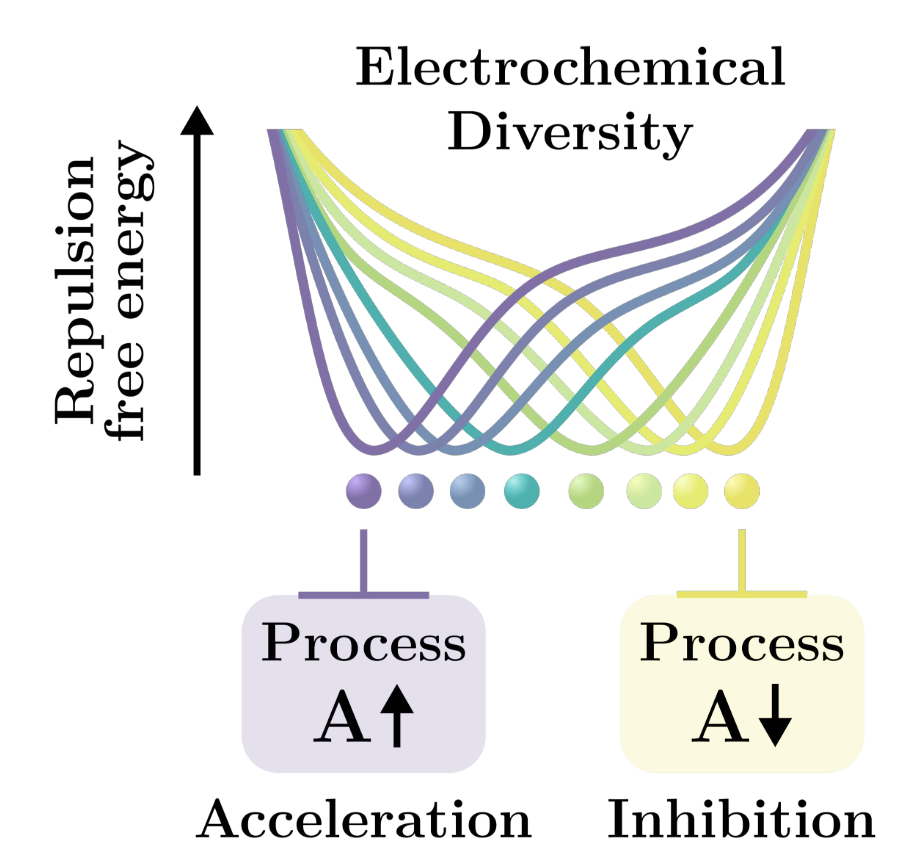 Biomolecular condensates sustain pH gradients at equilibrium driven by charge neutralisationHannes Ausserwoeger, Rob M. Scrutton, Tomas Sneideris, Charlotte M. Fischer, and 14 more authorsbioRxiv, 2024
Biomolecular condensates sustain pH gradients at equilibrium driven by charge neutralisationHannes Ausserwoeger, Rob M. Scrutton, Tomas Sneideris, Charlotte M. Fischer, and 14 more authorsbioRxiv, 2024Electrochemical gradients are essential to the functioning of cells and are typically formed across membranes using active transporters and require energy input to maintain them. Here, we show by contrast that biomolecular condensates are able to sustain significant pH gradients without any external energy input. We explore the thermodynamic driving forces that establish this gradient using a microfluidics-based droplet platform that allows us to sample in a continuous manner both the stability and composition of the condensates across a wide pH range. These results reveal that condensed biomolecular systems adjust the pH of the dense phase towards the isoelectric point (pI) of the component polypeptide chains. We demonstrate, on the basis of two representative systems, FUS and PGL3, that condensates can create both alkaline and acidic gradients with a magnitude exceeding one pH unit. Investigations of multicomponent protein/nucleic acid systems further show that heterotypic interactions can modulate condensate pH gradients. We further investigate using a bioinformatics approach the diversity of electrochemical properties of complex condensates by studying a large set of human condensate networks, showing that these span a wide range of mixture pIs and pH-response behaviours. In summary, our results reveal that protein condensation may present a fundamental physico-chemical mechanism for the effective segregation and optimisation of functional processes through changes in the emergent electrochemical microenvironment.Competing Interest StatementT.P.J.K. is the C.T.O. of Transition Bio Inc., G.I. and N.L.Z. are employees of Novo Nordisk and S.A and A.A.H. are members of the scientific advisory board of Dewpoint Therapeutics Inc. The work reported here was not influenced by these affiliations.
-
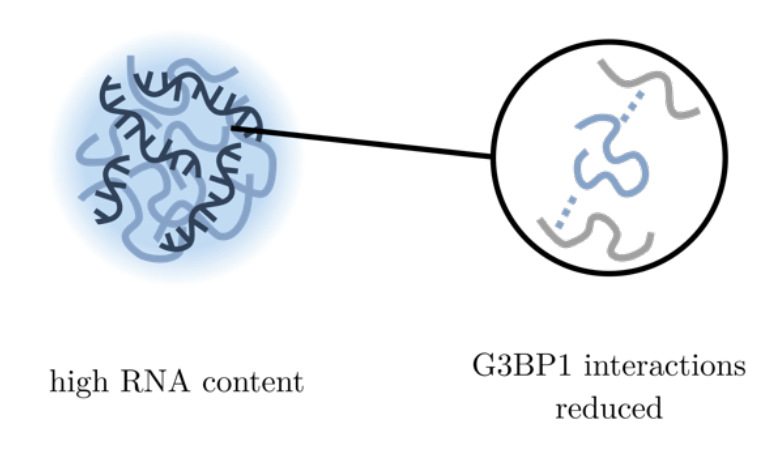 Temperature-induced changes in protein interactions control RNA recruitment to G3BP1 condensatesCharlotte M. Fischer, Hannes Ausserwöger, Tomas Sneideris, Daoyuan Qian, and 4 more authorsbioRxiv, 2024
Temperature-induced changes in protein interactions control RNA recruitment to G3BP1 condensatesCharlotte M. Fischer, Hannes Ausserwöger, Tomas Sneideris, Daoyuan Qian, and 4 more authorsbioRxiv, 2024Biomolecular condensates have emerged as prominent regulators of dynamic subcellular organisation and essential biological processes. Temperature, in particular, exerts a significant influence on the formation and behaviour of biomolecular condensation. For example, during cellular heat stress, stress granules (SGs) are formed from RNA-binding proteins (RBPs) and RNA, forming liquid condensates to protect the RNA from damage. However, the molecular mechanisms leading to changes in protein phase behaviour are not well understood. To answer how temperature modulates protein interactions and phase behaviour, we developed a high-throughput microfluidic platform, capable of mapping the phase space and quantifying protein interactions in a temperature-dependent manner. Specifically, our approach measures high-resolution protein phase diagrams as a function of temperature, while accurately quantifying changes in the binodal, condensate stoichiometry and free energy contribution of a solute, hence, providing information about the underlying mechanistic driving forces. We employ this approach to investigate the effect of temperature changes on the phase separation of the stress granule scaffold protein Ras GTPase-activating protein-binding protein 1 (G3BP1) with PolyA-RNA. Surprisingly, we find that the G3BP1/RNA phase boundary remains unaffected by the increasing temperature but the underlying stoichiometry and energetics shift, which can only be revealed with high-resolution phase diagrams. This indicates that temperature-induced dissolution is counteracted by entropic processes driving phase separation. With increasing temperature, the G3BP1 content in condensates decreases alongside with a reduction of the free energy of protein interactions, while the RNA content increases driven by entropically favoured hydrophobic interactions. In the context of cellular heat SG formation, these findings could indicate that during heat shock, elevated temperatures directly induce RNA recruitment to stress granules as a cytoprotective mechanism by finetuning the strength of protein and RNA interactions.Competing Interest StatementThe Authors declare the following competing interests: T.P.J.K. and P.S.G.-H. are a co-founders and H.A., D.Q., R.S. and S.Q. are employees or consultants for Transition Bio; C.M.F. and T.S. declare no competing interests.
2023
-
 Linking modulation of bio-molecular phase behaviour with collective interactionsDaoyuan Qian, Hannes Ausserwoger, William E. Arter, Rob M. Scrutton, and 9 more authorsbioRxiv, 2023
Linking modulation of bio-molecular phase behaviour with collective interactionsDaoyuan Qian, Hannes Ausserwoger, William E. Arter, Rob M. Scrutton, and 9 more authorsbioRxiv, 2023Bio-molecular condensates formed in the cytoplasm of cells are increasingly recognised as key spatiotemporal organisers of living matter and are implicated in a wide range of functional or pathological processes. This opens up a new avenue for condensate-based applications and a crucial step in controlling this process is to understand the underlying interactions driving their formation or dissolution. However, these condensates are highly multi-component assemblies and many inter-component interactions are present, rendering it difficult to identify key drivers of phase separation. In this work, we employ the recently formulated dominance analysis to modulations of condensate formation, centred around dilute phase solute concentration measurements. We posit that mechanisms of action of modulators can be categorised into 4 generic classes with respect to a target solute, motivated by theoretical insights. These classes serve as a general guide towards deducing possible mechanisms on the molecular level, which can be complemented by orthogonal measurements. As a case study, we investigate the modulation of suramin on condensates formed by G3BP1 and RNA, and the dominance measurements point towards a dissolution mechanism where suramin acts on G3BP1 to disrupt G3BP1/RNA interactions, as confirmed by a diffusional sizing assay. Our approach and the dominance framework have a high degree of adaptability and can be applied in many other condensate-forming systems.